How Radon Gas Is Formed
The Natural Radio Active Decay in Soil
How Radon Gas Is Formed Through Natural Radioactive Decay In Soil
Radon gas emerges from a natural process deep within Earth's crust, affecting homes and buildings worldwide. Radon forms when uranium and other radioactive elements in rocks and soil break down through radioactive decay, releasing this invisible and odorless gas into the surrounding environment.
We encounter radon regularly, as it seeps up through foundation cracks and accumulates in buildings. This radioactive gas ranks as the second leading cause of lung cancer in the United States, making its formation and presence relevant to our health and safety.
The formation of radon connects directly to Earth's geological composition, with some areas producing higher concentrations than others based on their underlying rock formations. Our location's geology plays a crucial role in determining our potential exposure to this naturally occurring radioactive gas.
What Is Radon Gas?
Radon gas is a naturally occurring radioactive element that exists as a colorless, odorless, and tasteless noble gas. It poses significant health risks when it accumulates in enclosed spaces, particularly in buildings and homes.
Physical and Chemical Properties
Radon (atomic number 86) belongs to the noble gas family on the periodic table. At room temperature, it maintains a gaseous state and has a density of 9.73 g/L, making it about eight times heavier than air.
Its chemical symbol is Rn, and it has a boiling point of -61.7°C and a melting point of -71°C.
As a noble gas, radon exhibits low chemical reactivity and rarely forms compounds with other elements.
Radioactive Nature of Radon
Radon-222, the most common isotope, has a half-life of 3.82 days. During radioactive decay, it emits alpha particles and transforms into polonium-218.
This decay process continues through several daughter products, creating a chain of radioactive elements.
The alpha particles released during radon decay can damage lung tissue when inhaled, making it a significant health concern.
Prevalence in the Environment
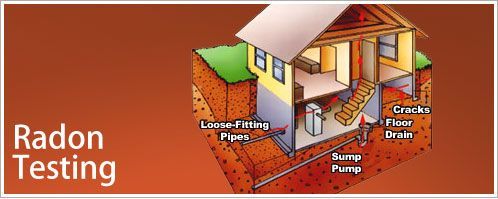
Radon forms naturally from the breakdown of uranium in soil, rock, and water. It seeps through cracks in foundations and accumulates in basements and lower floors of buildings.
Concentration levels vary significantly by geographic location, depending on local geology and soil composition.
We find the highest radon levels in areas with uranium-rich bedrock or granite formations.
Common entry points include:
- Foundation cracks
- Construction joints
- Gaps around service pipes
- Sump pumps and drains
The Formation Of Radon Gas
Radon gas emerges through a complex series of nuclear transformations within the Earth's crust. The process involves specific radioactive elements breaking down into lighter elements through natural decay chains.
Natural Radioactive Decay Process
Radioactive decay transforms heavier elements into lighter ones through the emission of particles and radiation. When an atom's nucleus becomes unstable, it releases energy and subatomic particles to achieve stability.
During this process, the atomic structure changes as protons and neutrons are ejected from the nucleus. Each step in the decay chain produces different radioactive isotopes.
The formation of radon occurs near the end of these decay chains. As atoms decay, they emit alpha particles, beta particles, or gamma radiation.
Role of Uranium and Thorium
Uranium-238 and thorium-232 serve as the primary parent isotopes in radon formation. These elements exist naturally in rocks, soil, and minerals throughout Earth's crust.
The concentration of uranium and thorium varies by geographical location. Areas with granite, shale, or phosphate deposits typically contain higher levels of these elements.
We find uranium-238 most abundantly in nature, making up 99.3% of all uranium on Earth. Its decay chain produces radon-222, the most common radon isotope.
Creation of Radium as a Precursor
Radium forms as an intermediate step in the decay chains of uranium and thorium. Each type of radium isotope produces a different radon isotope.
Radium-226 decays directly into radon-222 with a half-life of 1,600 years. The process releases an alpha particle, transforming the radium atom into a radon atom.
The conversion happens continuously in soil and rock. The newly formed radon atoms can move through tiny spaces between soil particles and enter the air or groundwater.
Geological Sources of Radon
Radon emerges from specific geological formations and materials that contain naturally occurring radioactive elements, primarily uranium-238.
Granite and Other Radioactive Rocks
Granite serves as a primary source of radon gas due to its high uranium content. When uranium within granite decays, it produces radium-226, which then transforms into radon gas.
Other igneous rocks like pumice and basalt also contain varying concentrations of uranium and thorium, contributing to radon production.
We find that metamorphic rocks, particularly those formed from uranium-rich sediments, can release significant amounts of radon through their fractures and crystal boundaries.
Soil Composition and Mineral Content
Sandy soils permit faster radon movement due to their high permeability and large pore spaces between particles.
Clay-rich soils typically contain higher concentrations of uranium and radium, making them substantial radon sources. These soils can trap the gas more effectively due to their dense structure.
Phosphate deposits and mineral-rich soils often contain elevated levels of uranium, creating localized radon hotspots.
Influence of Earth's Crust
The thickness and composition of bedrock layers directly affect radon migration patterns. Faults and fractures in the Earth's crust create pathways for radon to travel upward.
Tectonic activity can expose uranium-bearing rocks closer to the surface, increasing radon emissions in specific geographical regions.
Areas with complex geological histories often show varied radon concentrations due to the mixing and layering of different rock types over time.
Migration and Accumulation Mechanisms
Radon gas moves through different pathways in the environment, driven by physical forces and environmental conditions that influence its concentration levels in buildings and water sources.
Movement Through Soil and Rocks
Radon travels through soil and rock formations via two primary mechanisms: diffusion and advection. Diffusion occurs when radon moves from areas of high concentration to low concentration.
Pressure differences in soil create advective flow, pushing radon through pore spaces and fractures in rocks. The soil's permeability greatly affects how easily radon can move through it.
Weather conditions impact radon movement, with changes in atmospheric pressure and soil moisture affecting its migration rates. Dry, permeable soils allow for faster radon transport than water-saturated ground.
Entry Into Buildings and Structures
Foundation cracks and gaps serve as primary entry points for radon gas. The stack effect creates negative pressure in buildings, actively drawing radon from the soil below.
Common entry points include:
- Floor-wall joints
- Foundation cracks
- Sump pump basins
- Utility penetrations
- Porous building materials
Building ventilation patterns and HVAC systems influence radon accumulation rates. Lower floors typically show higher concentrations due to their proximity to soil sources.
Waterborne Radon Transport
Groundwater dissolves and carries radon through aquifers and water-bearing rock formations. Wells that tap into radon-rich groundwater can bring the gas directly into homes.
Surface water typically contains lower radon levels since the gas easily escapes into the air. Private wells in granite areas face higher risks of radon contamination.
Water use activities like showering and dishwashing release dissolved radon into indoor air. The transfer rate from water to air depends on water temperature and agitation levels.
Factors Affecting Radon Gas Levels
Multiple elements influence radon concentrations in buildings and outdoor spaces, from natural geological features to human modifications of the environment.
Geographical Variations
Radon levels vary significantly based on local geology and soil composition. Areas with uranium-rich granite bedrock typically show higher radon concentrations.
Soil permeability plays a crucial role in radon movement. Sandy soils allow radon to travel more freely than clay-based soils.
Elevation and topography affect radon distribution. Valley locations often experience higher radon accumulation due to temperature inversions trapping the gas near ground level.
Seasonal and Environmental Influences
Temperature differences between indoor and outdoor air create pressure variations that draw radon into buildings. This effect intensifies during winter months when homes are sealed tight.
Precipitation levels impact soil moisture content, which can block or release radon gas. Wet soil tends to trap radon, while dry conditions allow easier movement.
Barometric pressure changes affect radon migration through soil. Low pressure systems can pull more radon from the ground into buildings.
Human Activity and Land Use
Construction methods and building materials significantly impact indoor radon levels. Concrete foundations with cracks or gaps provide entry points for the gas.
Mining operations and ground disturbance can release trapped radon. These activities often expose new rock surfaces, increasing radon emissions.
Building ventilation systems and air exchange rates directly influence indoor radon accumulation. Poor ventilation leads to higher concentrations.
Changes in land use, such as soil compaction or removal of surface vegetation, can alter natural radon pathways in the ground.
Detection and Measurement of Radon Formation
Accurate measurement of radon gas requires specialized equipment and standardized protocols to identify radiation levels in buildings and soil. Testing methods vary from short-term snapshots to long-term monitoring systems that provide detailed data about radon concentrations.
Sampling Techniques
Short-term testing uses activated charcoal devices placed in buildings for 2-7 days. These collectors absorb radon particles from the air for laboratory analysis.
Long-term testing spans 90+ days using alpha track detectors or electret ion chambers. This method provides more representative results by accounting for seasonal variations.
Continuous monitoring employs electronic devices that record radon levels in real-time. We place these units in basements or ground floors where radon typically accumulates.
Soil gas sampling involves extracting air samples from probe holes at various depths. This technique helps identify radon entry points and geological sources.
Instrumentation and Devices
Active Devices:
- Continuous radon monitors
- Working level monitors
- Ion chamber detectors
Passive Devices:
- Charcoal canisters
- Alpha track detectors
- Electret ion chambers
Electronic devices measure alpha particle emissions through semiconductor sensors. These provide instant readings and data logging capabilities.
Laboratory analysis equipment includes gamma spectroscopy systems and liquid scintillation counters. These tools process collected samples with high precision.
Interpretation of Findings
The EPA action level for indoor radon is 4 pCi/L (picocuries per liter). Readings above this threshold indicate the need for mitigation measures.
We measure uncertainty factors and calculate statistical confidence levels for each testing method. Quality control procedures ensure accurate results.
Geographic and seasonal patterns affect measurement interpretation. Winter readings often show higher concentrations due to reduced ventilation.
Test results guide decisions about mitigation strategies and help identify high-risk areas within structures.
Environmental and Health Considerations
Radon gas poses significant risks to human health and requires careful monitoring in both indoor and outdoor environments to protect public safety.
Persistence in the Atmosphere
Radon gas remains active in the atmosphere for 3.8 days before it breaks down through radioactive decay. This half-life allows the gas to accumulate in enclosed spaces like basements and crawl spaces.
The gas can travel through soil and enter buildings through foundation cracks, utility penetrations, and construction joints. Air pressure differences between indoor and outdoor environments create a vacuum effect that draws radon inside structures.
Weather patterns and seasonal changes affect radon concentrations. Cold weather and closed windows typically lead to higher indoor levels as ventilation decreases.
Potential Health Impacts
Radon exposure is the second leading cause of lung cancer in the United States, after smoking. The radioactive particles can damage lung tissue when inhaled.
An estimated 21,000 lung cancer deaths occur annually in the US due to radon exposure. The risk increases significantly for smokers exposed to elevated radon levels.
Children face higher risks due to their developing bodies and faster breathing rates. Long-term exposure, even at moderate levels, can lead to serious health consequences.
Regulatory Guidelines
The EPA recommends taking action when indoor radon levels reach 4 picocuries per liter (pCi/L) or higher. Professional mitigation is advised at these concentrations.
Many states require radon testing during real estate transactions. Licensed professionals must perform testing and mitigation according to established protocols.
Building codes in high-risk areas often mandate radon-resistant construction features. These include vapor barriers, proper sealing, and sub-slab ventilation systems.
Regular testing is essential, as radon levels can change over time due to geological shifts and building modifications.
Reducing Exposure to Naturally Formed Radon
Effective radon control measures combine proper ventilation systems with specialized building techniques to minimize indoor radon concentrations to safe levels below 4 picocuries per liter (pCi/L).
Mitigation Strategies
A radon testing kit helps identify dangerous concentration levels in homes. Professional testing services can provide detailed measurements across different areas of the building.
Installing a soil suction system beneath the foundation draws radon from the soil before it enters living spaces. These systems use PVC pipes and fans to vent the gas safely above the roofline.
Sealing foundation cracks and openings with polyurethane caulk or epoxy prevents radon from seeping inside. We recommend inspecting these seals annually.
Key mitigation methods:
- Sub-slab depressurization
- Basement pressurization
- Crack sealing
- Sump pit covers
Building Design and Ventilation
New construction should incorporate radon-resistant features from the start. A layer of gravel beneath the foundation slab allows gases to move freely toward collection points.
Proper ventilation design includes dedicated intake and exhaust vents. We recommend installing mechanical ventilation systems that maintain neutral or positive air pressure inside the building.
Essential design elements:
- Vapor barriers under slabs
- Sealed foundation joints
- Airtight ductwork
- Multiple air exchanges per hour
Regular maintenance of ventilation systems ensures optimal performance. Clean filters and functioning fans help maintain proper airflow patterns that prevent radon accumulation.
We would be happy to discuss how we can help to reduce the risk in your home. Call us to set up a consultation